Over 4,000 providers across the U.S. are empowered to easily and affordably target dermatological conditions for their patients.
Enhance adherence by offering combined ingredients in one medication.
Boost overall efficiency and profitability by eliminating the administrative burden of managing insurance claims, allowing staff to focus more on patient care.
Spare patients from waiting in pharmacy lines and avoid medication substitutions to ensure they receive the medication they deserve at a more accessible price.
Choose medications without potentially harmful inactive ingredients and allergens commonly found in comparable branded and generic drugs*.
SKNV’s hassle-free prescribing experience increases patient loyalty and drives referrals. Additionally, we help you target core skin conditions. Depending on the needs of your practice and applicable state regulations, SKNV medications are available through two main models:
You can purchase inventory, immediately provide the medication to your patient at the time of their visit, and monetize your practice, all while offering a great experience to your patient.
You can prescribe from your EMR to send our customized medications directly to your patient’s home with no sales tax (in most states) and no shipping cost to your patients.
Branded & Generic Medicines
may contain inactive ingredients that are potential allergens or irritants for certain patients, as determined by the provider*
SKNV
medications without potentially harmful inactive ingredients and allergens, for certain patients and as determined by prescribers
SKNV
pharmacy for sensitive skin nationwide
SKNV
patients prescribed SKNV Rx every month for their sensitive skin
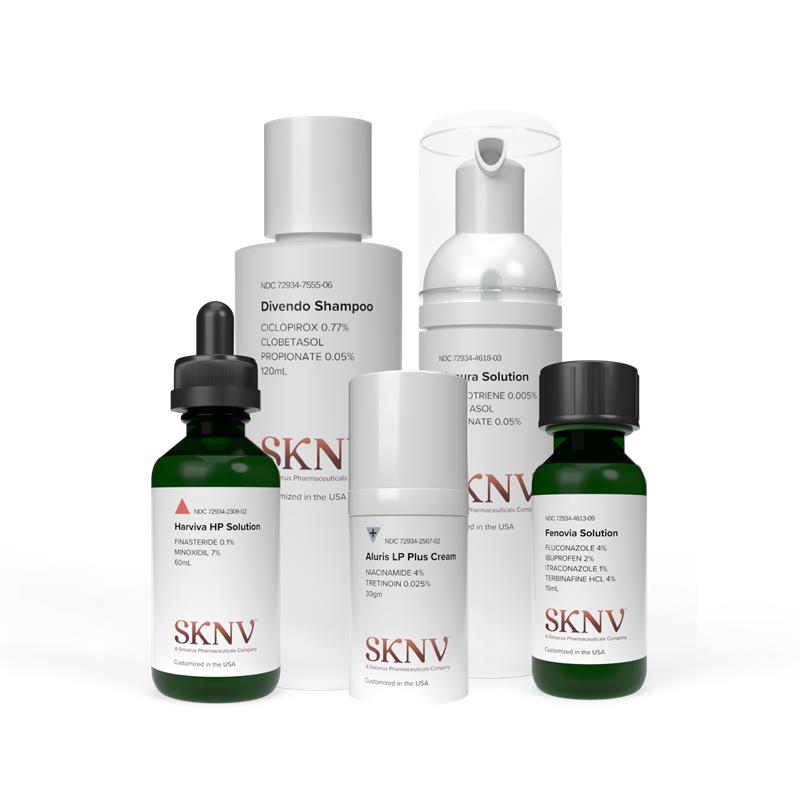
SKNV was born from the realization that the U.S. insurance industry was failing dermatology patients.
SKNV provides reliable medications at a great price point. Following stringent quality assurance measures, our formulations are tailored to your skin needs and without potential harmful excipients found in comparable branded and generic drugs, for certain patients as determined by the provider.*
Spencer Malkin, SKNV CEO and Co-Founder
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Websites store cookies to enhance functionality and personalise your experience. You can manage your preferences, but blocking some cookies may impact site performance and services.
Essential cookies enable basic functions and are necessary for the proper function of the website.
Google Tag Manager simplifies the management of marketing tags on your website without code changes.
These cookies are used for managing login functionality on this website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Clarity is a web analytics service that tracks and reports website traffic.
Service URL: clarity.microsoft.com
SourceBuster is used by WooCommerce for order attribution based on user source.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.
Facebook Pixel is a web analytics service that tracks and reports website traffic.
Service URL: www.facebook.com
LinkedIn Insight is a web analytics service that tracks and reports website traffic.
Service URL: www.linkedin.com
TikTok Pixel is a tracking tool that measures user interactions and optimizes ad campaigns on the TikTok platform.
Service URL: ads.tiktok.com
You can find more information in our Terms and Conditions and Terms and Conditions.