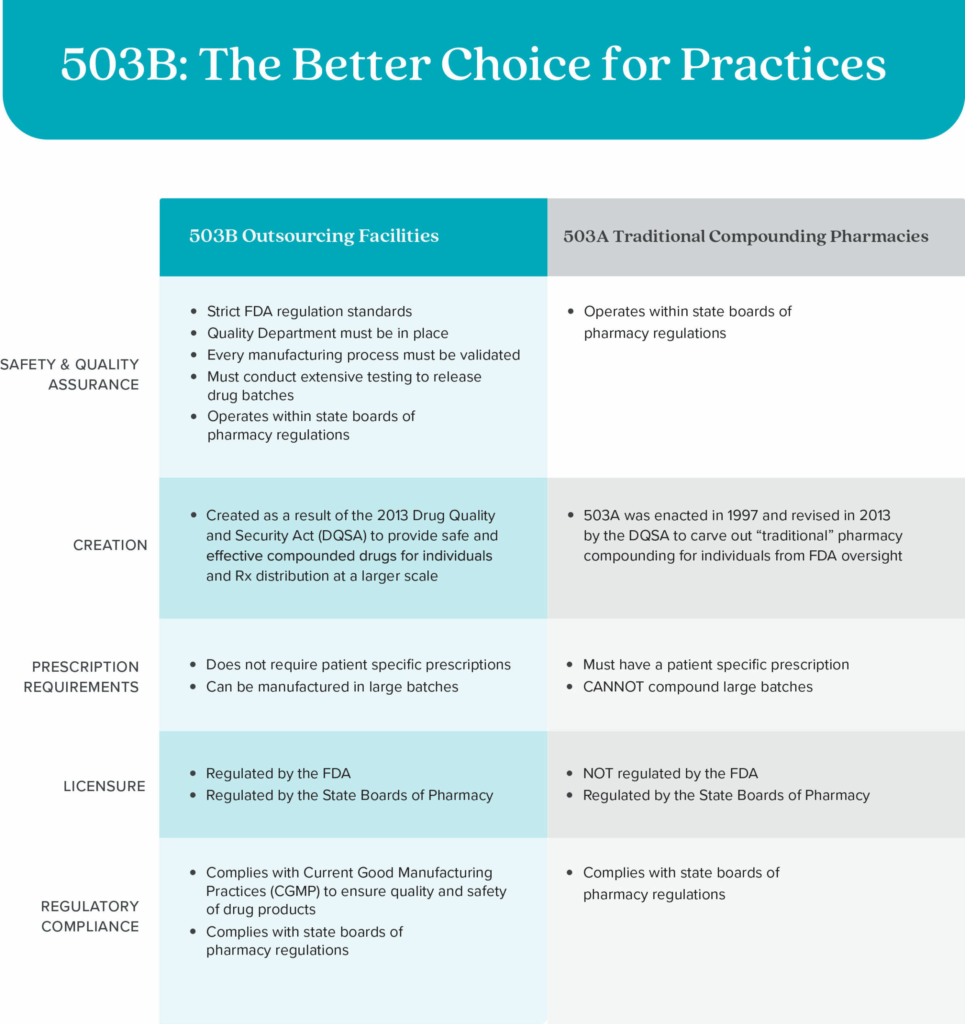
503B Outsourcing Facilities are not the same as 503A Compounding Pharmacies. 503A Compounding Pharmacies are not registered or regularly inspected by the FDA, cannot compound in large batches, and do not allow practices to dispense from their office.
In 1938, Congress passed a set of laws giving authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of food, drugs, and cosmetics. Dubbed the Federal Food, Drug, and Cosmetic Act (FD&C Act), this gave the legal framework within which the FDA operates.
In 2013, a 503A company, New England Compounding Center, caused a tragic and deadly meningitis outbreak due to their contaminated medications. This major incident highlighted significant lapses in the quality control and safety standards of compounded drugs.
After this incident, Congress passed the Drug Quality and Security Act (DQSA), which amended the FD&C Act to grant the FDA more authority to regulate and monitor the manufacturing of compounded drugs. This legislation aimed to prevent future tragedies by ensuring higher standards of safety and quality in the compounding pharmacy industry. A new category of FDA-regulated companies was born, and this is why SKNV was founded.
We use cookies to improve your experience on our site. By using our site, you consent to cookies.
Websites store cookies to enhance functionality and personalise your experience. You can manage your preferences, but blocking some cookies may impact site performance and services.
Essential cookies enable basic functions and are necessary for the proper function of the website.
Google Tag Manager simplifies the management of marketing tags on your website without code changes.
These cookies are used for managing login functionality on this website.
Statistics cookies collect information anonymously. This information helps us understand how visitors use our website.
Clarity is a web analytics service that tracks and reports website traffic.
Service URL: clarity.microsoft.com
SourceBuster is used by WooCommerce for order attribution based on user source.
Marketing cookies are used to follow visitors to websites. The intention is to show ads that are relevant and engaging to the individual user.
Facebook Pixel is a web analytics service that tracks and reports website traffic.
Service URL: www.facebook.com
LinkedIn Insight is a web analytics service that tracks and reports website traffic.
Service URL: www.linkedin.com
TikTok Pixel is a tracking tool that measures user interactions and optimizes ad campaigns on the TikTok platform.
Service URL: ads.tiktok.com
You can find more information in our Terms and Conditions and Terms and Conditions.